Magnesium stearate is a magnesium salt combined with stearic acid. Magnesium is an essential mineral that it is often missing from the human diet despite the many places it is found. It helps to keep blood sugar and blood pressure at the right levels. It helps form DNA and protein, and keeps muscles and nerves functioning correctly. Women need 320 mg/day whilst men need 420 mg/day to be healthy. Stearic acid is a common fatty acid found in fish, grains, eggs, butter, poultry, meat and dairy products. In meat, it makes up one third of the saturated fat. So it is a common, safe ingredient that is already familiar to consumers, which is important for consumer confidence.
Magnesium stearate is a fine white powder. Its main purpose is providing an effective dry lubricant for capsules and tablets. It increases flowability, which ensures efficiency through the manufacturing process. It is inert and can make your formulation process much easier, making it one of the most popular flowing agents in the industry.
Lubricants are crucial in tableting for specific processing benefits. There are three important roles of a lubricant:
- To lessen friction at the junction between a tablet's surface and the die wall during ejection, reducing wear on the tablets.
- To prevent the solution from sticking to the parts of a machine that it is currently running through.
- To increase flow by reducing friction amongst the particles themselves.
A good lubricant will form a strong layer over the surface. Another requirement is that it is adverse to a changes that may be caused by process variables such as flow, pressure and temperature. If any of these changes occur, the manufacturing process needs to remain unaffected.
Magnesium stearate is:
- "GRAS" (Generally Recognized As Safe) listed
- Designated as a food additive in Europe
- Manufactured from vegetables oils, most commonly palm oil and cottonseed oil
Stearates can be manufactured through hydrogenation (treatment with hydrogen). The result of this process is a product that needs to be handled with care.
Magnesium stearate cannot be mixed with air as dust explosion can occur. It should be kept away from flames at all costs. If it is dry, it can be charged electrostatically by swirling and pouring among other actions. If inhaled it can cause coughing, and if ingested vomiting may occur. Use protective gloves, safety goggles and a mask while handling this product.
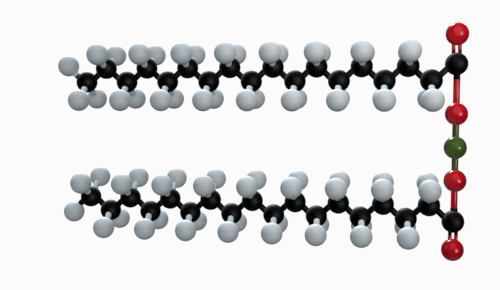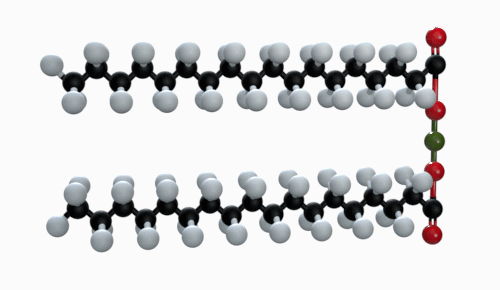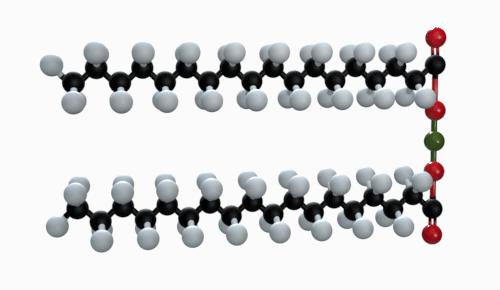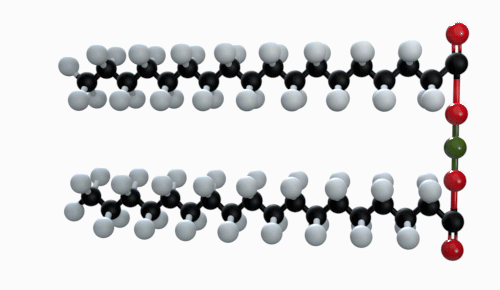
The chemical formula of magnesium stearate is Mg(C18H35O2). Other names for magnesium stearate are octadecanoic acid and magnesium salt.
This product is generally the top choice as a dry lubricant. Additionally, as a mineral comfortable in the human body it can be consumed safely on a regular basis. It might be the perfect excipient for your product.